Product
Western Blots & Immunoassays
BlockPRO™ Protein Free Blocking Buffer is a non-protein formulation which enhances sensitivity and minimizes background noise, presenting better results than traditional protein-based blocking buffer in immunoassays. The synthetic formulation of BlockPRO™ Protein-Free Blocking Buffer makes it suitable for PVDF and nitrocellulose platform, avidin/biotin system, detection of phosphoprotein, and other immunochemical applications.
Highlights:
- High performance: Provide better specific signal and less background noise than traditional blocking buffer
- Multiple application: Suitable for Western blot, dot blot, ELISA, and other immunoassays
- High quality control: Ensure lot-to-lot consistency for your most reproducible results over time
Order Information:
| Cat. No. | Product Name | Description |
| BF01-1L | BlockPRO™ Protein-Free Blocking Buffer |
500mL Solution X 2 |
| BF10-100 | BlockPRO™ Protein-Free Blocking Buffer (10X) |
100mL 10X Solution X 1 |
| BF10-200 | BlockPRO™ Protein-Free Blocking Buffer (10X) |
100mL 10X Solution X 2 |
Product Detail:
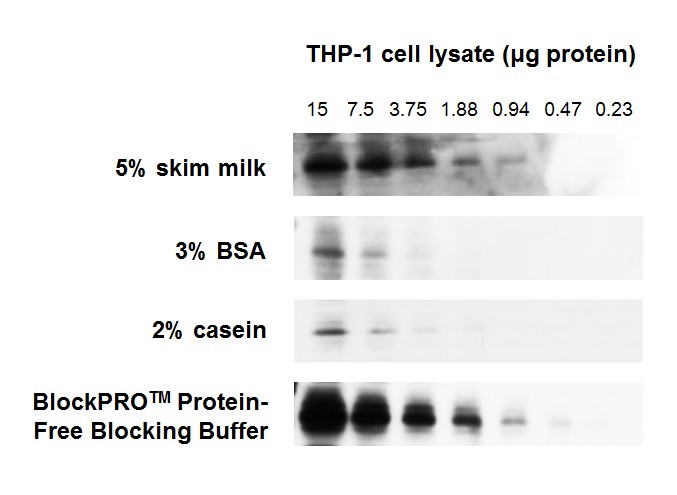
Figure 1. BlockPRO™ Protein-Free Blocking Buffer is better than protein-based blocking buffers (skim milk, BSA and casein) for detection of target protein in Western blotting.
THP-1 cell lysates were prepared and separated by electrophoresis. The proteins were transferred to PVDF and blocked for 1 hour at room temperature with the indicated blocking buffer, probed with mouse anti-pAMPK followed by anti-mouse HRP and detected by chemiluminescence. All results were exposed to X-ray film for 30 seconds.
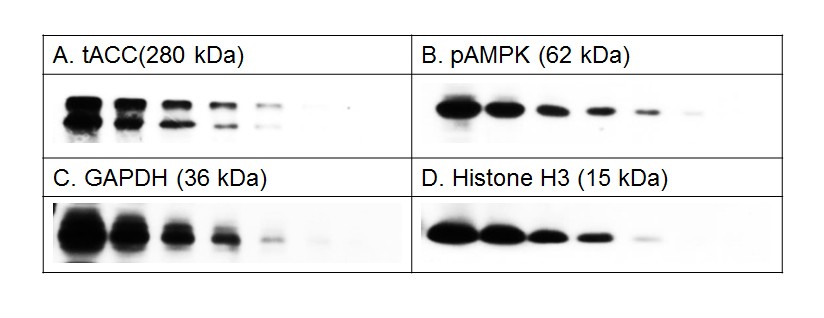
Figure 2. BlockPRO™ Protein-Free is suitable for various protein antigen detection, such as high molecular weight protein, tACC; phosphoprotein, pAMPK; abundant protein, GAPDH; low molecular weight protein, histone H3.
THP-1 cell lysates were prepared and separated by electrophoresis. The proteins were transferred to PVDF and blocked for 1 hour at room temperature with BlockPRO™ Protein-Free Blocking Buffer. Antibodies designed to probe the indicated proteins were used. All the signals were detected by chemiluminescence and were exposed to X-ray film.
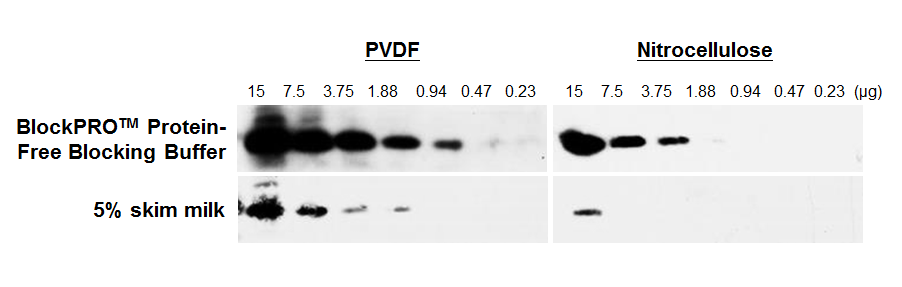
Figure 3. BlockPRO™ Protein-Free Blocking Buffer can be used in both PVDF and nitrocellulose platform.
Hela cell lysates were prepared and separated by SDS-PAGE. The proteins were transferred to PVDF or nitrocellulose membranes. The membranes were blocked for overnight at 4 °C with BlockPRO™ Protein-Free Blocking Buffer or 5% skim milk, probed with mouse anti-histone H3 followed by anti-mouse HRP and detected by chemiluminescence. All results were exposed to X-ray film for 30 seconds.
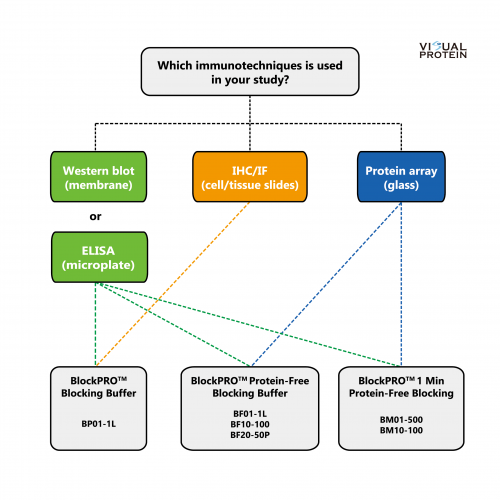
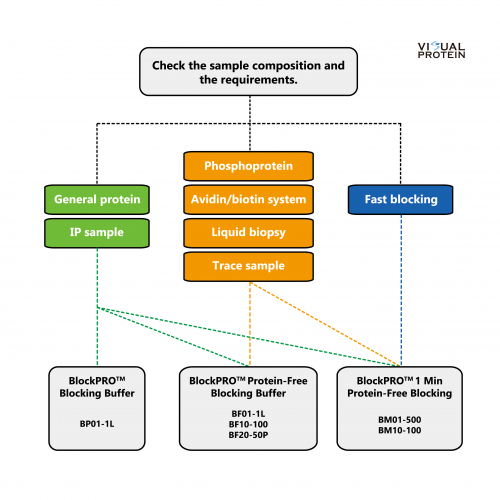
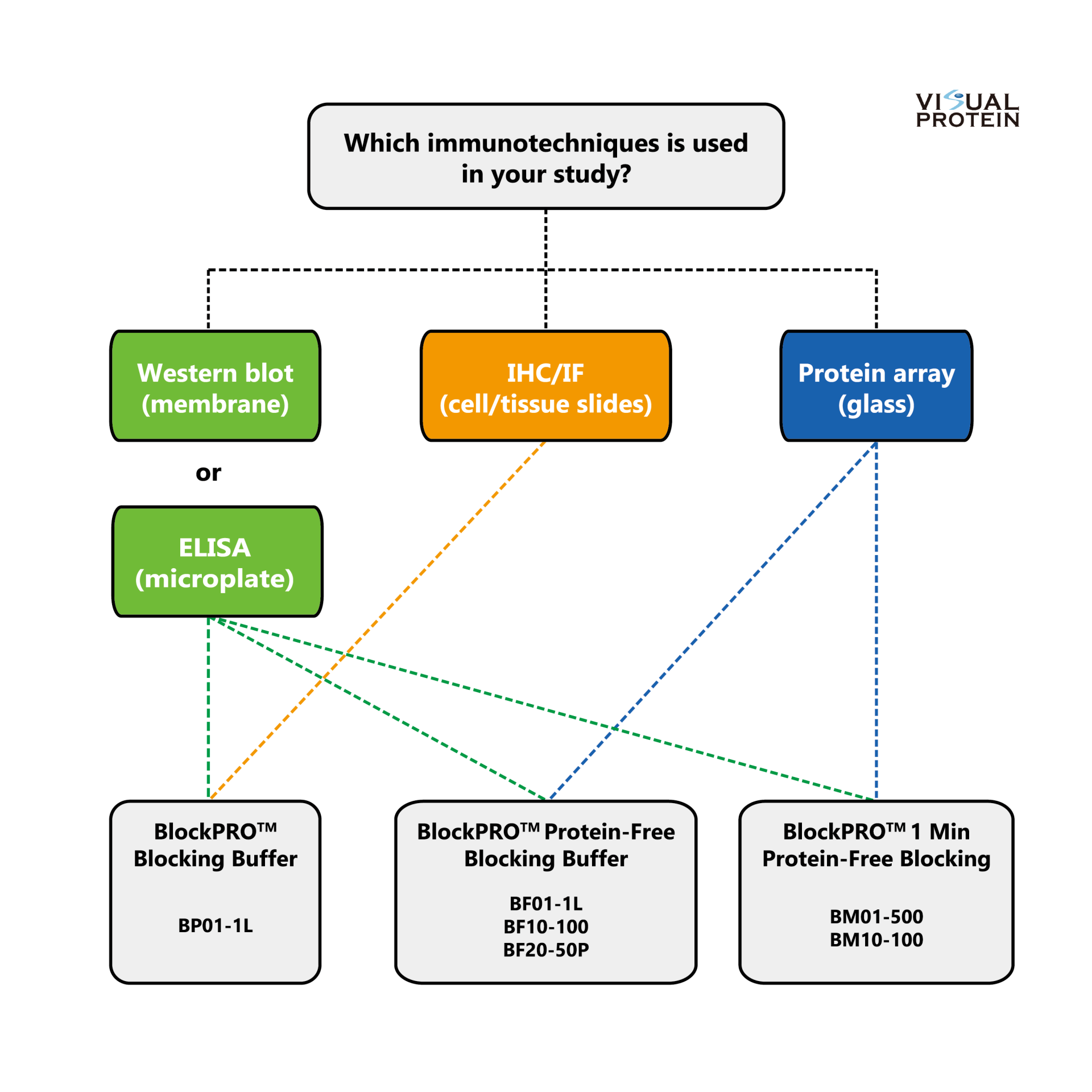
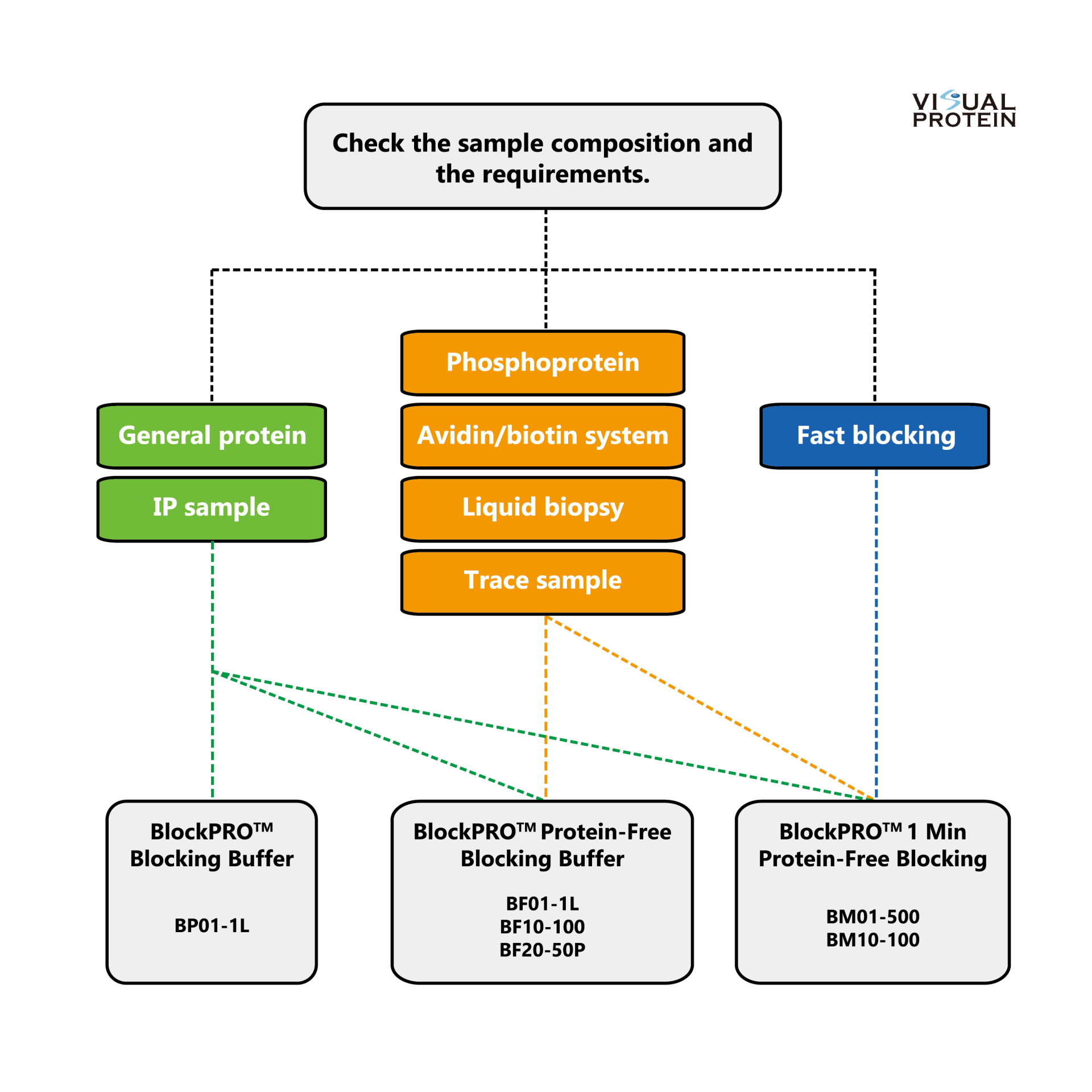
Selection Guide:
Reference:
| Item | Description | Application | Link |
| 1 | Scheidler, Christopher M., Milan Vrabel, and Sabine Schneider. "Genetic Code Expansion, Protein Expression, and Protein Functionalization in Bacillus subtilis." ACS Synthetic Biology 9.3 (2020) | ELISA | PMID: 32053368 |
| 2 | Chen, KY et al. Monascin accelerates anoikis in circulating tumor cells and prevents breast cancer metastasis. Oncol Lett. 2020 Nov;20(5):166. doi: 10.3892/ol.2020.12029. Epub (2020) | WB | PMID: 32934733 |
| 3 | Chang, Shih‐Chieh, et al. "Significant association of serum autoantibodies to TYMS, HAPLN1 and IGFBP5 with early stage canine malignant mammary tumours." Veterinary and Comparative Oncology 19.1 (2021) | ELISA | PMID: 33038064 |
| 4 | Wang, Ruike, et al. “High-throughput immunosensor chip coupled with a fluorescent DNA dendrimer for ultrasensitive detection of cardiac troponin T.” RSC Advances 11.44 (2021) | Immunosensor | PMID: 35480665 |
| 5 | Lin, Chi-Chien, et al. "Crassolide Suppresses Dendritic Cell Maturation and Attenuates Experimental Antiphospholipid Syndrome." Molecules 26.9 (2021) | WB | PMID: 33923336 |
| 6 | Tang, Kuo-Tung, et al. "Kurarinone Attenuates Collagen-Induced Arthritis in Mice by Inhibiting Th1/Th17 Cell Responses and Oxidative Stress." International journal of molecular sciences 22.8 (2021) | WB | PMID: 33924467 |
| 7 | Zhang, Man-Li, Wei-Wei Liu, and Wei-Dong Li. “Imbalance of Molecular Module of TINCR-miR-761 Promotes the Metastatic Potential of Early Triple Negative Breast Cancer and Partially Offsets the Anti-Tumor Activity of Luteolin.” Cancer Management and Research 13 (2021) | WB | PMID: 34654432 |
| 8 | Yuan, Stephen Hsien-Chi, et al. "Serum Level of Tumor-Overexpressed AGR2 is Significantly Associated with Unfavorable Prognosis of Canine Malignant Mammary Tumors." Animals 11.10 (2021) | ELISA, IF | PMID: 34679944 |
| 9 | Horikawa, Mei, Hisataka Sabe, and Yasuhito Onodera. “Dual roles of AMAP1 in the transcriptional regulation and intracellular trafficking of carbonic anhydrase IX.” Translational Oncology 15.1 (2022) | WB | PMID: 34742153 |
| 10 | Mishima, Satomi, et al. "Multifunctional regulation of VAMP3 in exocytic and endocytic pathways of RBL-2H3 cells." Frontiers in Immunology 13 (2022) | WB | PMID: 35990647 |
| 11 | Cheng, Wen-Chien, et al. "Pendulone induces apoptosis via the ROS-mediated ER-stress pathway in human non-small cell lung cancer cells." Toxicology in Vitro 81 (2022) | WB | PMID: 35288263 |
| 12 | Lu IT, Lin SC, Chu YC, et al. (-)-Agelasidine A Induces Endoplasmic Reticulum Stress-Dependent Apoptosis in Human Hepatocellular Carcinoma. Mar Drugs. (2022) | WB | PMID: 35200638 |
| 13 | Sung, Wei-Wen, et al. "Exopolysaccharides of Bacillus amyloliquefaciens Amy-1 mitigate inflammation by inhibiting ERK1/2 and NF-κB pathways and activating p38/Nrf2 pathway." International Journal of Molecular Sciences 23.18 (2022) | WB | PMID: 36142159 |
| 14 | Hirano-Maeda, Yuki, Daisuke Ojima, and Masaei Kanematsu. "Molecular characterization of Vasa homolog in the pen shell Atrina pectinata: cDNA cloning and expression analysis during gonadal development." Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 263 (2023) | WB | PMID: 36064136 |
| 15 | Sheu, Ming-Jen, et al. "Glucosinolates Extracts from Brassica juncea Ameliorate HFD-Induced Non-Alcoholic Steatohepatitis." Nutrients 15.16 (2023) | WB | PMID: 37630688 |
| 16 | Cherkashin, Alexander P., et al. "Contribution of TRPC3-mediated Ca2+ entry to taste transduction." Pflügers Archiv-European Journal of Physiology (2023) | WB | PMID: 37369785 |
| 17 | Puangpila C, Anukulkich N, Chiapleam S, Intajan B, Jakmunee J, Pencharee S. Development of lectin-based lateral flow assay for fucosylated alpha-fetoprotein. J Cell Biochem. 2023 Oct;124(10):1546-1556. | Lateral flow assay | PMID: 37665725 |
| 18 | Thanongsaksrikul, Jeeraphong, et al. "Characterization of antibody response to SARS-CoV-2 Orf8 from three waves of COVID-19 outbreak in Thailand." PloS one 19.5 (2024) | ELISA | PMID: 38768163 |
| 19 | Oikawa, Tsukasa, et al. "p53 ensures the normal behavior and modification of G1/S-specific histone H3. 1 in the nucleus." Life Science Alliance 7.9 (2024) | WB | PMID: 38906678 |
| 20 | Hu, Cheng-Ti, et al. "Strategic Design of Biocompatible, Glistening-Free, and Foldable Artificial Intraocular Lenses Based on Hydro-Amphiphilic Ternary Copolymers." Biomacromolecules (2025). | WB | PMID: 40526039 |
| 21 | Ting, Hui-Kung, et al. "Pyr3 inhibits cell viability and PKCα activity to suppress migration in human bladder cancer cells." European Journal of Pharmacology 988 (2025): 177235. | WB | PMID: 39725132 |
| 22 | Kamloon, Thitiporn, et al. "Exploring putative histone deacetylase inhibitors with antiproliferative activity of chrysin derivatives." Medicinal Chemistry Research 34.6 (2025): 1308-1320. | WB | LINK |
| 23 | Chung, Hsien-Hui, Ching-Jiunn Tseng, and Pei-Wen Cheng. "The multifaceted modulations by dapagliflozin in preventing hyperglycemia-induced diabetic nephropathy through glucose transporters and PPARα." Journal of Diabetes & Metabolic Disorders 24.2 (2025): 154. | WB | PMID: 40548192 |